Advisor: Dr. Pelegri
Lab Members involved: Mohit Agarwal
Abstract:
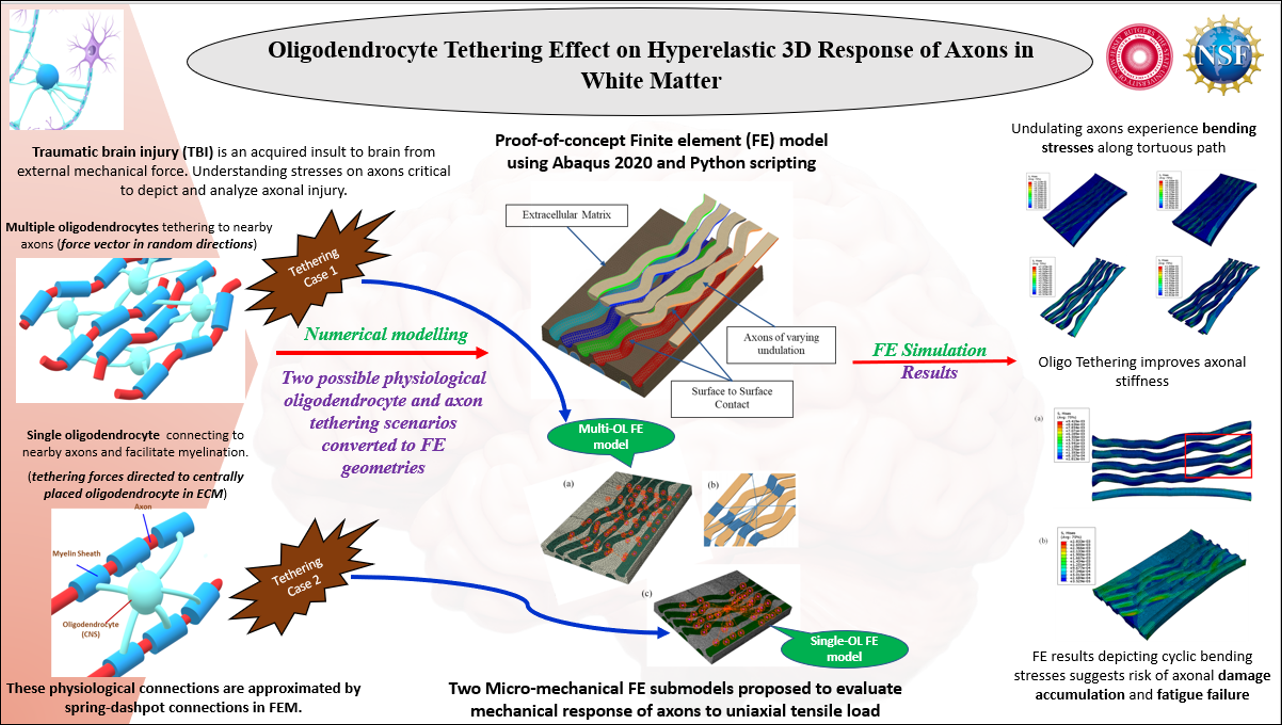
This research investigates the effects of oligodendrocyte tethering on the mechanical response of axons
in white matter under tensile loads. Novel finite element (FE) models using Ogden hyperelastic (HE) and
hyper-viscoelastic (HVE) material models are developed to simulate axons embedded in the extracellular
matrix. Two submodels are studied: a single oligodendrocyte (single-OL) model and a multi-
oligodendrocyte (multi-OL) model. In the multi-OL configuration, forces are randomly oriented due to
the arbitrary wrapping of glial cells around axons. In the single-OL setup, a centrally located
oligodendrocyte myelinates multiple axons, directing forces towards it and resulting in a more
directional stress distribution. The oligodendrocyte-axon connections are represented by a spring-
dashpot model to accurately determine stress states and axonal stiffness.
The FE models highlight the potential to estimate axonal injuries and the risk of damage accumulation
and fatigue failure due to cyclic bending stresses. Additionally, the hyper-viscoelastic models, analyzed
using single-OL submodels, depict the effects of strain rate and strain history due to repetitive uniaxial
stretching. Simulations include steady-state dynamic (SSD) and explicit dynamics (ED) cases. Results
indicate that bending stresses arise along axons' tortuous paths, with stress reversal due to inherent
tortuosity. The magnitude of these stresses depends on factors like axonal geometry, brain mass,
loading direction and frequency, and the state of shear moduli.
Prony Series parameters model strain-rate effects in SSD simulations, revealing that increased
oligodendrocyte tethering (higher spring stiffness and more connections) aids in stress relaxation,
reducing axonal stiffness over time. This behavior underscores the importance of oligodendrocyte
tethering in stress redistribution and tissue softening under repeated loads. The proposed SSD and ED
HVE models can be extended to evaluate the structural response of aging or injured axons in future
research.